Molecule
- Name
- Formic acid
- IUPAC name
- Formic acid
- Secondary names
- Methanoic acid, Aminic acid, Formylic acid, Hydrogen carboxylic acid, Hydroxymethanone, Hydroxy(oxo)methane, Metacarbonoic acid, Oxocarbinic acid, Oxomethanol, HCOOH, CH2O2, HCO2H
- InChI
- InChI=1S/CH2O2/c2-1-3/h1H,(H,2,3)
- InChI key
- BDAGIHXWWSANSR-UHFFFAOYSA-N
- CAS number
- 64-18-6
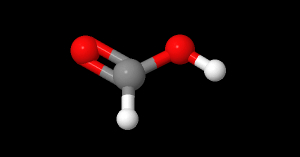
- Formula
- $HCOOH$
- Chemical formula
- CH2O2
- Structural formula
- [CH](=O)[OH]
- Charge
- 0
- Unpaired electrons
- 0
- Relations
-
- no
- Isotope mixture type
- terrestrial abundance
- Spin type
- equilibrated
- Molar mass
- 46.0254 $g/mol$
- State STP
- liquid
- State NTP
- liquid
- Protic
- protic
- Polarity
- polar
- Dipole
- 1.425 $D$
- Molecule symmetry
- Cs
- Case
- nonlinear-polyatomic
- Vibrations number
- 9
- Vibrations
-
Label Mode Bond Symmetry Degeneracy Fundamental frequency Observed frequency Observed frequency Harmonic frequency Activity IR Activity Raman Strength Comments V1 stretching $-O-H$ $A_{}'$ no 3570.0 3570.0 active m V2 stretching $\eqslantgtr C-H$ $A_{}'$ no 2943.0 2942.8 active m V3 stretching $>C=O$ $A_{}'$ no 1770.0 1770.0 active vs V4 bending $\eqslantgtr C-H$ $A_{}'$ no 1387.0 1387.0 active vw V5 bending $-O-H$ $A_{}'$ no 1229.0 1229.0 active w V6 stretching $\eqslantgtr C-O-$ $A_{}'$ no 1105.0 1105.3 active s V7 deformation $O=C(-)-O-$ $A_{}'$ no 625.0 625.0 active m V8 bending $\eqslantgtr C-H$ $A$ no 1033.0 1033.0 active w V9 deformation $HCOOH$ $A$ no 638.0 638.0 active s - Publications