Molecule
- Name
- Ammonia
- IUPAC name
- (14N,1H3)Ammonia
- Secondary names
- Azane, NH3, NH3-14
- InChI
- InChI=1S/H3N/h1H3/i1+0/hH3
- InChI key
- QGZKDVFQNNGYKY-OGMQTYGCSA-N
- Comments
- $^{14}NH_3$ isotope
- Formula
- $^{14}NH_3$
- Chemical formula
- NH3
- Isotopic formula
- [14]N[1]H3
- Structural formula
- [1H][14N]([1H])[1H]
- Charge
- 0
- Unpaired electrons
- 0
- Relations
-
- no
- Isotope mixture type
- pure isotope
- Spin type
- equilibrated
- Spin quantum numbers
- 1/2, 3/2
- Equilibrium meta/para ratio
- 1 (1/1)
- Molar mass
- 17.02655 $g/mol$
- State STP
- gas
- Protic
- protic
- Polarity
- polar
- Dipole
- 1.4718 $D$
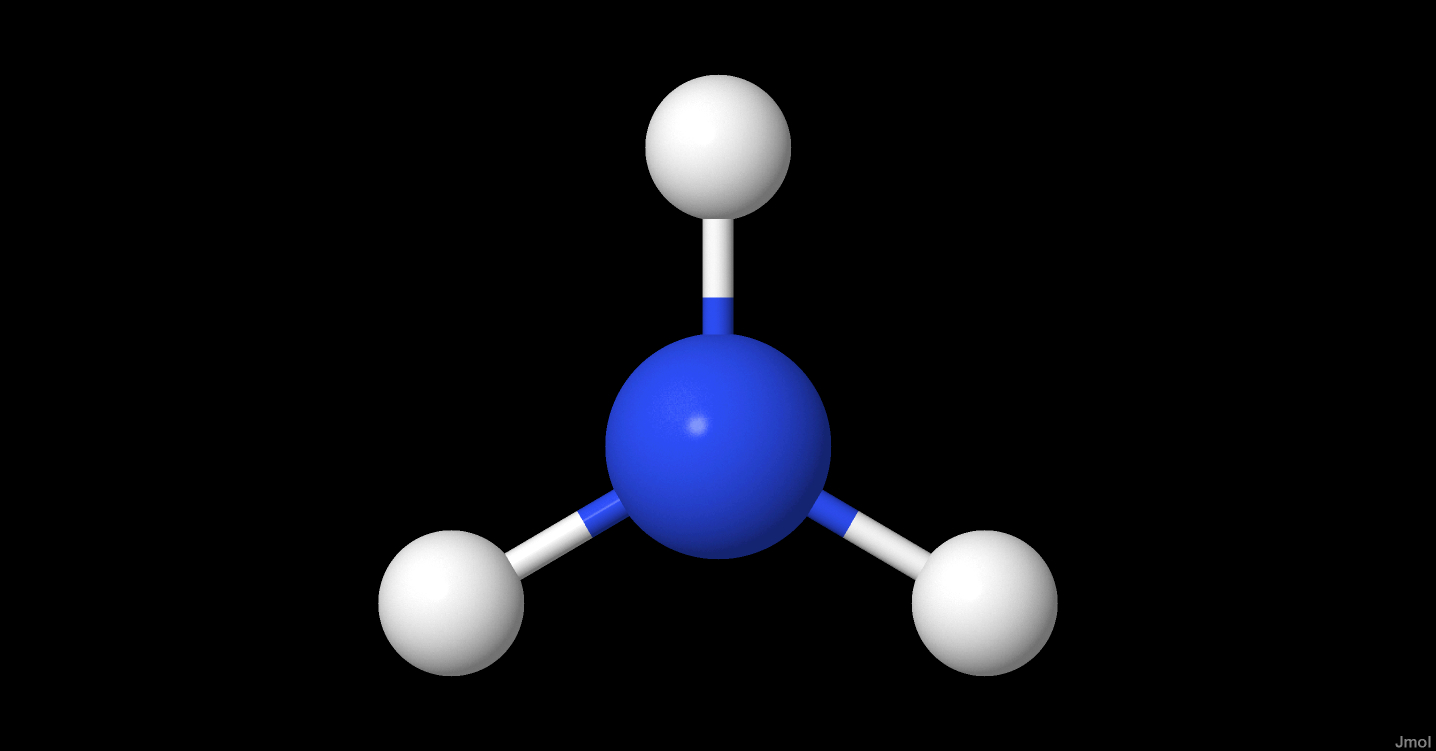
- Molecule symmetry
- C3v
- Case
- symmetric-top
- Vibrations number
- 4
- Vibrations
-
Label Mode Bond Symmetry Degeneracy Fundamental frequency Observed frequency Observed frequency Harmonic frequency Activity IR Activity Raman Strength Comments V1 stretching sym. $^{14}NH_3$ $A_{1}$ no 3337.0 3336.2 3337.2 3505.7 active active s observed split between symmetric and antisymmetric levels V2 bending sym. $^{14}NH_3$ $A_{1}$ no 950.0 935.5 968.3 1022.0 active active vs observed split between symmetric and antisymmetric levels V3 stretching $^{14}NH_3$ $E$ double 3444.0 3443.6 3443.9 3573.1 active active s observed split between symmetric and antisymmetric levels V4 deformation $^{14}NH_3$ $E$ double 1627.0 1626.1 1627.4 1689.7 active active vs observed split between symmetric and antisymmetric levels - Comments
- Data from NIST Chemistry WebBook and Benedict & Plyler (1957)
- Publications