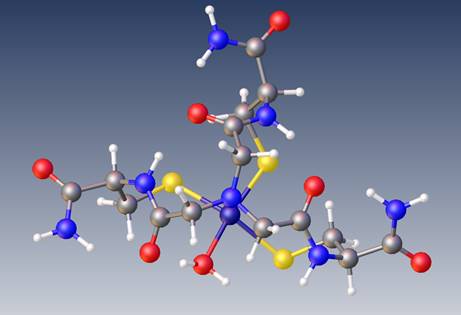
Molecule
- Name
- Ni NTA(CysNH2)3
- IUPAC name
- NONE
- Formula
- $N[CH_2(CONH)CH(CONH_2)CH_2S]_3Ni^{+} \cdot H_2O^{-}$
- Chemical formula
- N(C5N2H8O2S)3NiH2O
- Charge
- 0
- Unpaired electrons
- 0
- Relations
-
- no
- Isotope mixture type
- terrestrial abundance
- Spin type
- equilibrated
- Molar mass
- 571.3 $g/mol$
- State NTP
- liquid
- Protic
- protic
- Polarity
- polar
- Molecule symmetry
- unknown
- Case
- complex