Solid phase
- Official name
- Carbon dioxide ice polymorphs
- Secondary names
- CO2 ice; dry ice; solid CO2
- Family
- molecular solid
- Class
- non polar molecular solid
- Compound type
- inorganic molecular solid
- Formula
- $CO_2$
- Chemical formula
- CO2
- Elemental formula
- C O2
- Isotope mixture type
- terrestrial abundance
- Class
- molecular solids with apolar molecules (01)
- Type
- with polyatomic inorganic apolar molecules (01.07)
- Polymorphs
-
- Carbon dioxide ice (Low pressure (0-12 GPa) - low temperature (< 800 K) phase)
- Phase name
- CO2 ice polymorphs
- Phase type
- crystalline
- Crystal system
- various
- Crystal class
- various
- Molar mass
- 44.01 $g/mol$
- State NTP
- gas
- Phase transitions
-
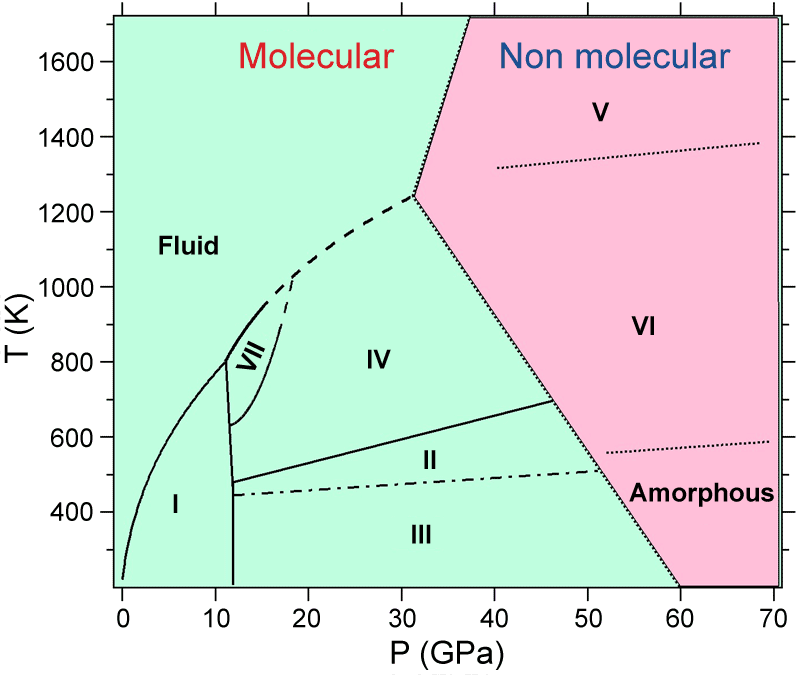
Phase transition type Other phases Temperature - Pressure triple point solid-liquid-gas $CO_2$-I ice -- liquid $CO_2$ - gas 216.58 K (5.185 bar) solid-liquid $CO_2$-I ice -- liquid $CO_2$ 216.58 K (0.52 MPa) - 800 K (~11 GPa) solid-solid $CO_2$-I ice -- $CO_2$-III ice < 445 K (~11.9 GPa) solid-solid $CO_2$-I ice -- $CO_2$-II ice 445 - 475 K (~11.9 GPa) solid-solid $CO_2$-I ice -- $CO_2$-IV ice 475 - 630 K (~11.6 - 11.9 GPa) solid-solid $CO_2$-I ice -- $CO_2$-VII ice 630 - 800 K (~11.2 - 11.6 GPa)
- Refringence type
- various
- Pure color
- colorless